WaPo Story on Drug Pricing Misses Opportunity to Talk Biosimilars
Last weekend, the Washington Post, in partnership with Kaiser Health News, published a news story on the issue of drug prices (what else?). Like many of these types of stories, it opens up with an example from a real-life patient.
The patient, Michael, was diagnosed with multiple sclerosis in 2016 and receives an infusion of Genentech’s Rituxan® (rituximab) every six months. As the article tells it, the price of Rituxan hovered between $6,201 and $6,841 for several years, with Michael’s insurance covering “most” of the cost. However, last fall, the cost of the drug increased to $10,320.
This sounds pretty terrible, right? Michael says it himself:
“Why does it have to increase in price all of a sudden?” said Costanzo, who lives in a small town about 50 miles north of Denver. “I think greed is a huge problem.”
Of course, it is not quite that easy. The article leaves out some key context:
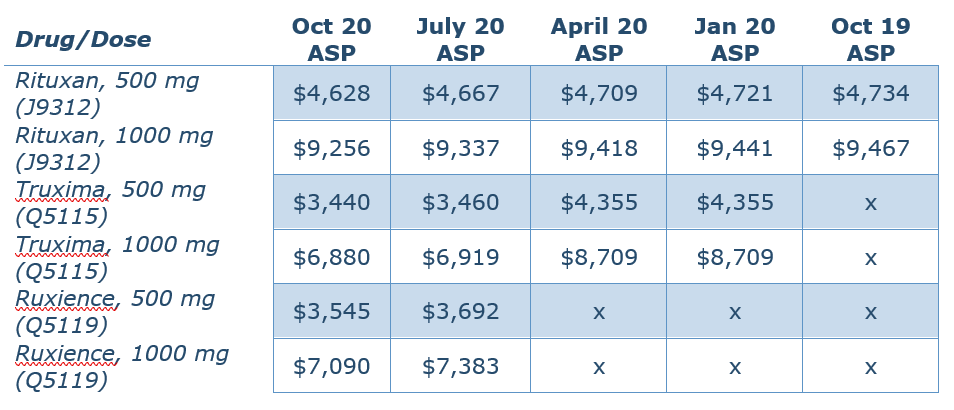
In November 2019, around the time of the price increase for Rituxan, Teva launched Truxima® (rituxan-abbs), a biosimilar competitor. In January 2020, Pfizer launched another biosimilar competitor, Ruxience® (rituximab-pvvr). Both products launched at a discount to Rituxan. I pulled the ASP pricing data from the Centers for Medicare and Medicaid Services (CMS) for all three agents:
Rituximab is used off-label for the treatment of multiple sclerosis (MS). This table uses a dose of 500 mg or 1,00 mg, based on this Neurology study. The Washington Post article does not specify which dose the patient takes.
Keep in mind that the U.S. Food and Drug Administration (FDA) has determined that the biosimilars Truxima and Ruxience are highly similar to and have no clinically meaningful differences in safety, purity, and potency (safety and effectiveness) from the reference product (in this case, Rituxan). And looking at the costs above, both Truxima and Ruxience are available at a significant discount to Rituxan.
So, an article on drug prices that appeared in a major national newspaper used an anecdote from a patient about their personal experience with drug prices, and left out a pretty big variable impacting that patient’s experience, namely the introduction of lower-cost, highly similar therapeutic options to the drug that has increased in cost.
Wow.
Of course, it’s not always that simple (it never is…). Maybe Michael’s insurance plan has blocked coverage of Truxima and Ruxience because of a rebate agreement negotiated with Genentech. This rebate may mean that the net cost to the plan for Rituxan is lower than either of the biosimilars. But it still leaves Michael with higher out-of-pocket costs, which appear to be calculated off the higher, non-rebated price.
Or maybe Michael’s plan covers both Rituxan and the biosimilars (or at least one of the biosimilars) but Michael’s physician is a preferred customer with Genentech and receives discounts that makes it financially advantageous from the practice’s point of view to continue to administer Rituxan over the biosimilars, and since Michael hasn’t expressed concern to the physician over his increased out-of-pocket costs, the physician hasn’t considered switching.
Or maybe Michael’s physician is not familiar with biosimilars or is otherwise weary of prescribing the products. So even though there are less expensive options available that have no clinically meaningful differences with the reference product, Michael is missing a huge opportunity to reduce costs for himself and his health plan by not giving the biosimilar a try.
My point is, we do not know, because the article does not examine any of these possibilities. Instead, the reporter uses this anecdote to illustrate a thesis on increasing drug prices, particularly for older drugs (the article immediately pivots to the insulin issue, which is also a unique problem). In reality, the story on this drug is more complicated.
Novacula Occamai, the reporter found this example, saw that it fit the thesis of the broader story, and has no idea that there is a more interesting story to tell here, with respect to this specific drug. What a missed opportunity to educate the public on some of the more complex issues facing the drug pricing debate. Sure, it is a lot more fun to just gang up on pharmaceutical manufacturers as bad actors and complain about increasing prices with little-to-no justification.
But it is critical that patients and the public at least realize the nuance in the broader drug policy debate. Without that, we run the risk of implementing policies that are not appropriately tailored to the realities of the marketplace and damaging the broader life sciences industry. Biosimilars offer the opportunity to introduce much-needed competition into the marketplace, but they have faced a lot of challenges in adoption. This article offered the opportunity to explore some of those challenges in-depth, and also to educate the public on the issues, but it missed.