New Model Would Recalibrate Medicare Payment for Top 50 Part B Drugs
The Trump Administration has released an interim final rule with comment period that implements a new model (run through the Center for Medicare and Medicaid Innovation, or CMMI) that will tie Medicare payment rates for the top 50 drugs paid under Medicare Part B to international prices. The rule is currently scheduled to take effect on January 1, 2021.
The model would run for seven years, from 2021 through 2027. CMMI would reevaluate the model prior to 2025 for impacts in beneficiary access to drugs or impacts on patient outcomes. Almost all Medicare providers would be included in the model; the Centers for Medicare and Medicaid Services (CMS) estimates that the model would impact 88% of Medicare spending on the included drugs.
The model would do the following:
Tie the Medicare reimbursement rate for included drug to the lowest price charged in one of 22 countries;
Establish an alternative add-on payment that is not linked to the overall cost of the drug; and
Reduce beneficiary cost-sharing.
CMS estimates that the model would reduce government expenditures and beneficiary costs by a total of $87.8 billion over seven years (because a reduction in beneficiary cost-sharing would involve cost-shifting back to the government, the net savings is lower than the aggregate):
$64.4 billion in reduced Medicare fee-for-service payments;
$49.6 billion in reduced Medicare Advantage plan payments;
$9.9 billion in reduced costs to states and the federal government under Medicaid; and
$28.5 billion in savings for beneficiaries, through lower Part B premiums and lower cost-sharing.
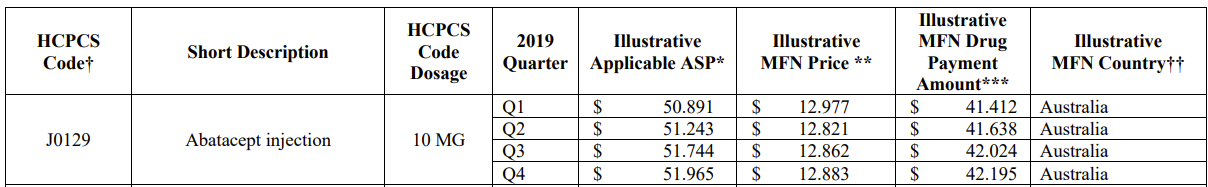
CMS has identified the top 50 drugs, by total Medicare spending, in 2019, for inclusion in the initial year of the model. Biosimilars will only be included in the model when spending on the biosimilar itself exceeds the threshold; they will be excluded otherwise. The new pricing structure will be phased in over the first three years of the model. As is now, prices will be updated on a quarterly basis, but drugs will only be considered for inclusion/exclusion in the model on an annual basis.
Illustrative pricing data for the drugs was included in the final rule (in Table 6 for those who want to CTRL+F). An example:
Unlike the rebate safe harbor rule that was also released today, this final rule may be challenged in court under the Administrative Procedure Act, since it did not go through the full notice-and-comment rulemaking process: instead the agency went from an advanced notice of proposed rulemaking straight to an interim final rule with no formal proposed rule. Congress may also be more willing to challenge the rule under the Congressional Review Act, since the “model” is mandatory and nationwide and therefore could overstep existing agency authority to change Medicare payment rates.
Absent action by the courts or Congress, it is not clear whether a Biden Administration would try to revoke the rule or leave it in place; initial reactions from Democrats indicate general support of the model. Any action under the CRA would require support from House Democrats and either Senate Republicans or Senate Democrats (depending on the outcome of the January 5, 2021 Georgia Senate run-offs).